The CEMB awards SEED Grants to faculty members from CEMB member institutions at the rank of assistant or associate professor who are not currently CEMB faculty fellows. See Guidelines for the SEED Grant program. This spring, six new grants were awarded:
Joel Boerckel, University of Pennsylvania, Read more…
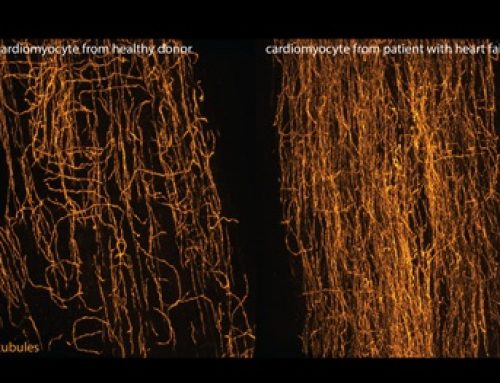
Cells are able to respond to a variety of mechanical stimuli, from fluid shear blood vessel cells, to the changing mechanical properties of the environment in which a cancer cell resides. Cells interpret these mechanical stimuli through a process called “mechanosensation” and translate them into the biochemical language of the cell through “mechanotransduction.” It is often assumed that the progression of mechanosensation to mechanotransduction is a one-way-street, but there is evidence that the biochemical products of mechanotransduction can also regulate how the cells perform mechanosensation. The goal of this project is to understand how these mechanotransductive feedback loops work.
ImageJ=1.50i
unit=micron
Deep Jariwala, University of Pennsylvania, Read more…
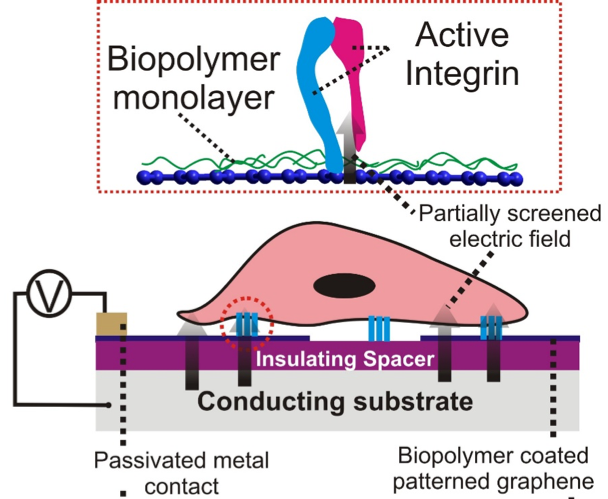
This SEED aims to probe cell-matrix adhesion by use of atomically thin conducting materials such as graphene sheets as interfacial layers. By spatially patterning and varying the conductivity of graphene sheets, the interactions of adhesion molecules such as integrin. More specifically the atomically thin nature of graphene allows electric-field to penetrate through it thereby having a potential to affect local adhesion of integrin which contains calcium or magnesium ions by application of electric voltage. A proof of concept demonstration will open doors to electrical control of cell adhesion, growth, and proliferation.
Celeste Morley, Washington University in St. Louis, Read more…
Specialized immune cells in the lung, called alveolar macrophages, control how much inflammation the lungs generate after inhalation of an irritant (like smoke) or a germ. Deciding how much inflammation to cause is important. If the lungs don’t generate enough inflammation, the body won’t be able to clear the irritant or germ. If the lungs generate too much inflammation, the healthy tissue can be damaged. Our laboratory studies a protein in the alveolar macrophage, called L-plastin, which helps the macrophages stick to the lung tissue. We propose that one way alveolar macrophages decide how much inflammation to cause is by sensing lung stiffness—healthy lungs are very soft, but diseased lungs get very stiff. We also propose that L-plastin is a protein that helps the macrophages sense lung stiffness. Our CEMB project investigates how L-plastin helps the alveolar macrophages sense stiffness and how sensing stiffness helps the macrophages decide how much inflammation to cause.
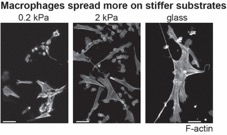
Amit Pathak, Washington University in St. Louis, Read more…
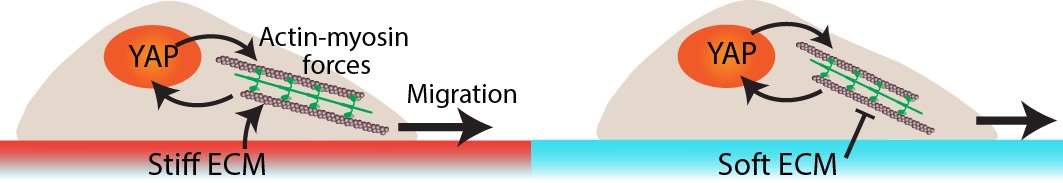
Collectively migrating cells sense matrix stiffness through adhesions and cytoskeletal forces. We have recently shown that mechanical priming of epithelial cells on a stiff ECM enhances their future migration, even after they move to a soft ECM. This storage of past mechano-activation in migratory cells depends on nuclear YAP localization. In this project, we investigate biophysical mechanisms that connect nuclear YAP to cellular forces and collective cell migration. Based on experimental findings, we are also building a computational framework for collective cell migration across matrices of dissimilar stiffness through rate-dependent mechanotransduction. Through this integrated approach, we aim to understand how mechanical priming and memory might be regulated by the crosstalk of nucleus shape, YAP localization, cytoskeletal machinery, and cell migration, all operating at multiple time and length scales.
Murat Guvendiren, New Jersey Institute of Technology, Read more…
Stem cell response to curvature with tunable stiffness and ligand expression – Stem cells reside in a complex and dynamic microenvironment, where they sense geometrical, chemical and mechanical cues, and respond to it. There is increasing evidence that curvature of the external environment, including at the scale of a cell length, directly affects stem cell behavior. Yet, existing cell culture systems fail to accurately mimic the architecture experienced by stem cells in 3D. This proposal will address this gap. The main goals of this proposal are: (i) to develop a fundamental understanding of the effects of “cell-scale” (on the order of a cell length) curvature on cell function, and (ii) to determine the effect of matrix stiffness and ligand density on the ability of a cell to sense and respond to curvature. We propose to develop 3D bioprinted hydrogel devices from novel bioink formulations that will enable unparalleled control over curvature, stiffness, and ligand expression.
Melike Lakadamyali, University of Pennsylvania, Read more…
One overarching goal of the Lakadamyali lab is to determine the interplay between epigenetics, the physical architecture of chromosomes and gene regulation. The human genome folds in a highly complex manner inside the nucleus and this folding is fundamentally important for regulating gene expression. However, how the genome is folded at the kilo-to-megabase genomic scales (10-300 nm spatial scale), which are the scales most relevant for gene regulation, has been difficult to determine since these length scales are inaccessible to conventional light microscopy. We have been overcoming this technical challenge by applying quantitative super-resolution microscopy methods to study the 3D organization of the genome in intact nuclei at the single cell level with nanoscale spatial resolution. We are determining how mechanical and chemical cues remodel the genome structure and how this remodeling regulates gene activity and cell fate determination.