Led by a Team of Multidisciplinary Experts
The leadership team at CEMB is committed to advancing the study of mechanical forces in molecules, cells and tissue in plants and animals as we train the new generation of scientific leaders. Learn more about our leadership team below.
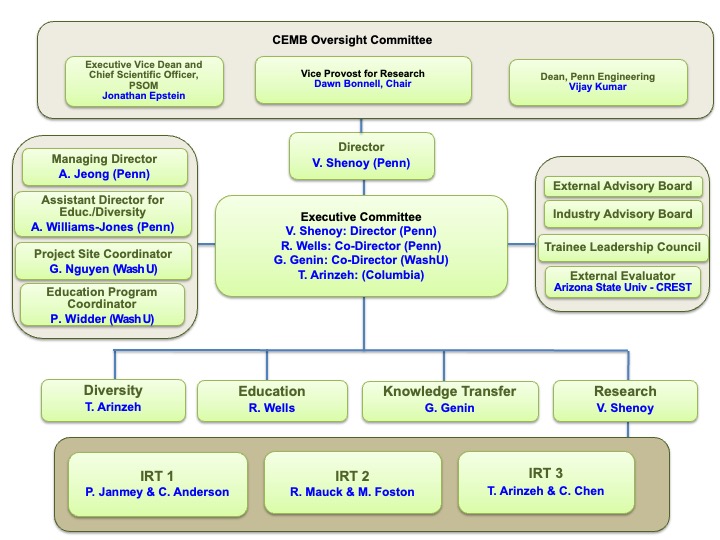
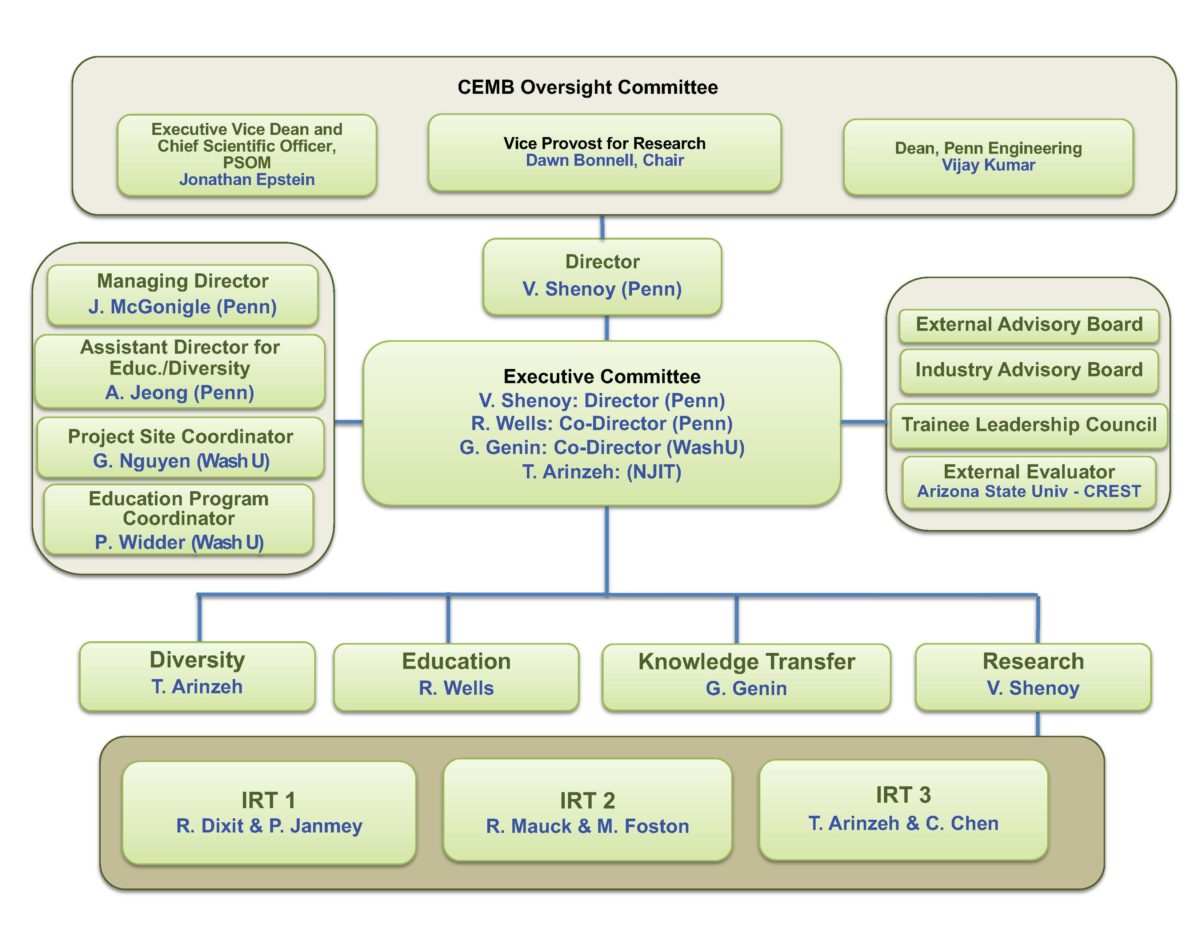
Executive Committee
Vivek Shenoy, PhD
Center Director and Director of Research
Vivek Shenoy, Ph.D., is the Eduardo D. Glandt President’s Distinguished Professor in the School of Engineering and Applied Science with appointments in the Departments of Materials Science and Engineering, Mechanical Engineering and Applied Mechanics, and Bioengineering. He is Director of the Center for Engineering MechanoBiology.
Professor Shenoy’s research team focuses on developing theoretical concepts and numerical methods to understand the basic principles that control the behavior of both engineering and biological systems. A significant challenge in modeling the engineering and biological systems examined by Shenoy’s team is that important processes involve the coupling of both small-scale (atomic or single molecule) phenomena and long-range (elastic, electromagnetic) interactions over length scales of hundreds of nanometers. His group addresses these issues by combining atomic scale simulation methods with continuum or mesoscale theories and by adapting insights from condensed matter physics, solid mechanics, chemistry, materials science and applied mathematics.
Keywords: Cell-matrix interactions, Chemo-mechanical models for cell adhesions and motility, Nuclear mechanotransduction, Mechanics of fibrous networks
Rebecca Wells, MD
Director of Education | IRT 1 Co-Leader
Rebecca Wells, M.D., is Professor of Medicine, Bioengineering, and Pathology and Laboratory Medicine with graduate area affiliations in Bioengineering, Cell and Molecular Biology, and Pharmacology at the University of Pennsylvania. She is the Director of Education of the Center for Engineering MechanoBiology.
The Wells Lab focuses on the mechanism and consequences of hepatic fibrosis. Overall, the goal of the research team is to develop a unified and comprehensive model of liver fibrosis that incorporates multiple cell types, soluble and secreted factors, matrix proteins, and local and regional mechanical factors.
Liver fibrosis results from the deposition of excess, abnormal extracellular matrix by myofibroblasts derived from non-fibrogenic cells that undergo “activation” in the context of chronic liver injury. Fibrosis in the bile duct is a similar matrix-driven process, although the identity of the myofibroblast populations and the chronic vs. acute nature of the injury are not known.
The Wells Lab is investigating the mechanisms of fibrosis in four ways: a) by studying the matrix, mechanical, and soluble factors that influence fibrosis, including the activation of myofibroblast precursor populations; b) by studying the interactions between proteoglycans and collagen as it relates to fibrotic diseases; c) by identifying new fibrogenic cell populations and new means of studying previously identified cells; and d) by applying the results of experiments with isolated cells to whole animal models and to the study of human diseases, including hepatocellular carcinoma, fatty liver disease, and biliary fibrosis.
Keywords: Hepatic stellate cells, liver fibrosis, TGF-ß, portal fibroblasts, biliary atresia, liver mechanics, fibronectin; mechanobiology
Guy Genin, PhD
Co-Director | Director of Knowledge Transfer
Guy Genin, Ph.D., is the Harold and Kathleen Faught Professor of Mechanical Engineering in the Department of Mechanical Engineering and Materials Science and a Professor of Neurological Surgery at Washington University in St. Louis. Genin is Chief Engineer for the Center for Innovation in Neuroscience and Technology. He also serves as the Chinese Ministry of Education Yangtze River Professor and McDonnell International Scholars Academy Ambassador to Xi’an Jiaotong University.
Genin is Co-Director of the Center for Engineering MechanoBiology, and the Primary Investigator of the WashU CEMB.
Genin’s research focuses on interfaces and adhesion in nature, physiology, and engineering. His current research focuses on interfaces between tissues at the attachment of tendon to bone, between cells in cardiac fibrosis, and between protein structures at the periphery of plant and animal cells.
Keywords: biophysical methods; biophysics; cell biology; extracellular matrix; substrates; periplasm configuration; cell adhesion and spreading; auxin; arabinogalactins; electrical signalling; mechanobiology
Treena Arinzeh, PhD
Director of Community | IRT 3 Co-Leader
Treena Livingston Arinzeh, PhD is a Professor of Biomedical Engineering at Columbia University. Dr. Arinzeh received her B.S. from Rutgers University in Mechanical Engineering, her M.S.E. in Biomedical Engineering from Johns Hopkins University, and her Ph.D. in Bioengineering from the University of Pennsylvania. She worked for several years as a project manager at a stem cell technology company, Osiris Therapeutics, Inc. Before moving to Columbia University, Dr. Arinzeh was faculty at NJIT as one of the founding faculty members of the department of Biomedical Engineering and served as interim chairperson and graduate director. Her most notable or cited work to date has been in the use of allogeneic mesenchymal stem cells (MSCs) with bioactive ceramics to induce bone formation in a large bone defect without the use of immunosuppressive therapy. This study served as the basis for FDA approval to pursue clinical trials using allogeneic MSCs for various applications.
Elizabeth Haswell, PhD
Elizabeth Haswell, Ph.D., is Professor of Biology at Washington University in St. Louis. She has been named a Faculty Scholar with the Simons Foundation and the Howard Hughes Medical Institute, and serves as Research Director for the Center for Engineering MechanoBiology. Liz is a Reviewing Editor for The Plant Cell and is currently a Guest Editor for Current Opinion in Plant Biology. She serves on the North American Arabidopsis Steering Committee, and the Multinational Arabidopsis Steering Committee, and in 2018 was elected as an AAAS council delegate. She is an advocate for science communication and for an academic culture that values sustainability, diversity, and authenticity. She is a co-host of the Plantae Taproot podcast.
The Haswell Lab aims to identify the molecular and cellular mechanisms by which land plants perceive force, with a particular focus on mechanosensitive ion channels. The Haswell group is currently studying the structure, function, regulation, and evolution of several families of mechanosensitive ion channels using live-imaging, single channel patch clamp electrophysiology, and complementary biochemical and molecular genetic approaches. They are also engaged in functional and genetic screens designed to identify novel mechanosensory proteins, and in the development of new tools for the non-invasive analysis of membrane forces.
Visit the Haswell Lab website.
Keywords: mechanosensitive ion channels, mechanosensitivity, cell signaling, programmed cell death, biochemical and molecular genetic approaches, live-imaging, electrophysiology; mechanobiology
Working Group(s): Working Group 1: How do cells sense their mechanical environment?; Working Group 2: How do cells adapt to and change their mechanical environment?; Working Group 3: How do cells remember their mechanical environment?; Working Group 4: Crosscutting and Emerging Technologies
IRT Leaders
Ram Dixit
IRT 1 Co-Leader
Ram Dixit is a Professor of Biology at Washington University in St. Louis and Associate Director of Education for the Center for Engineering MechanoBiology.
Dixit’s research seeks to understand the interplay between biochemical and mechanical stimuli that control the growth and morphology of plant cells. In plants, the noncentrosomal cortical microtubule cytoskeleton determines cell shape by spatially organizing the rigid cell wall which specifies the direction of turgor-driven cell expansion. Anisotropic expansion in turn feeds back to alter cortical microtubule organization. The Dixit lab wants to elucidate the molecular mechanisms that generate and dynamically modify the organization of the cortical microtubules during plant growth and development. We are also interested in understanding how the cortical microtubule array guides the ordered deposition of cell wall constituents to affect the structure and mechanics of the cell wall. The lab uses a multi-disciplinary and multi-scale approach combining live-imaging, in vitro reconstitution at the single-molecule level, molecular genetics and computational modeling.
Keywords: plant cell morphogenesis; cortical microtubule cytoskeleton; plant cell wall; mechanobiology; microtubule plus-end and minus-end targeting proteins; microtubule severing; microtubule bundling; microtubule self-organization; kinesins
Rebecca Wells, MD
Director of Education | IRT 1 Co-Leader
Rebecca Wells, M.D., is Professor of Medicine, Bioengineering, and Pathology and Laboratory Medicine with graduate area affiliations in Bioengineering, Cell and Molecular Biology, and Pharmacology at the University of Pennsylvania. She is the Director of Education of the Center for Engineering MechanoBiology.
The Wells Lab focuses on the mechanism and consequences of hepatic fibrosis. Overall, the goal of the research team is to develop a unified and comprehensive model of liver fibrosis that incorporates multiple cell types, soluble and secreted factors, matrix proteins, and local and regional mechanical factors.
Liver fibrosis results from the deposition of excess, abnormal extracellular matrix by myofibroblasts derived from non-fibrogenic cells that undergo “activation” in the context of chronic liver injury. Fibrosis in the bile duct is a similar matrix-driven process, although the identity of the myofibroblast populations and the chronic vs. acute nature of the injury are not known.
The Wells Lab is investigating the mechanisms of fibrosis in four ways: a) by studying the matrix, mechanical, and soluble factors that influence fibrosis, including the activation of myofibroblast precursor populations; b) by studying the interactions between proteoglycans and collagen as it relates to fibrotic diseases; c) by identifying new fibrogenic cell populations and new means of studying previously identified cells; and d) by applying the results of experiments with isolated cells to whole animal models and to the study of human diseases, including hepatocellular carcinoma, fatty liver disease, and biliary fibrosis.
Keywords: Hepatic stellate cells, liver fibrosis, TGF-ß, portal fibroblasts, biliary atresia, liver mechanics, fibronectin; mechanobiology
Melike Lakadamyali
One overarching goal of the Lakadamyali lab is to determine the interplay between epigenetics, the physical architecture of chromosomes and gene regulation. The human genome folds in a highly complex manner inside the nucleus and this folding is fundamentally important for regulating gene expression. However, how the genome is folded at the kilo-to-megabase genomic scales (10-300 nm spatial scale), which are the scales most relevant for gene regulation, has been difficult to determine since these length scales are inaccessible to conventional light microscopy. We have been overcoming this technical challenge by applying quantitative super-resolution microscopy methods to study the 3D organization of the genome in intact nuclei at the single cell level with nanoscale spatial resolution. We are determining how mechanical and chemical cues remodel the genome structure and how this remodeling regulates gene activity and cell fate determination.
Robert Mauck, PhD
IRT 2 Co-Leader
Robert Mauck, Ph.D., is the Mary Black Ralston Professor for Education and Research in Orthopaedic Surgery and Professor of Bioengineering at the University of Pennsylvania. He is the Director of the McKay Orthopaedic Research Laboratory and the Biomechanics Core of the Penn Center for Musculoskeletal Disorders. Dr. Mauck co-directs the Program in Musculoskeletal Regeneration in the Penn Institute for Regenerative Medicine. Mauck is a Research Health Scientist at the Philadelphia VA Medical Center, and co-Director of the Translational Musculoskeletal Research Center (TMRC) at the VA.
Mauck also serves Co-Leader of Working Group 3 for the Center for Engineering MechanoBiology.
The Mauck Lab research program in soft tissue mechanobiology and tissue engineering aims to understand the physiologic and patho-physiologic processes that regulate the formation, maturation, and adult function of soft connective tissues. His group focuses on the engineering of musculoskeletal tissues, with a particular interest in restoring articular cartilage, the knee meniscus, and the intervertebral disc. Dr. Mauck’s team uses rigorous mechanical and molecular analyses to characterize native tissue structure and function and employs this information to enhance the functional properties of engineered constructs through focused technology development. Specifically, this work employs adult mesenchymal stem cells, custom mechanobiologic culture conditions, and novel cell scaffolding technologies.
Keywords: soft tissue mechanobiology; physiologic processes; patho-physiologic processes; tissue engineering; technological development; mesenchymal stem cells; mechanobiologic cultures; cell scaffolding; mechanobiology
Christopher Chen
IRT 3 Co-Leader
Christopher Chen, M.D., Ph.D., is Professor of Biomedical Engineering and Materials Science Engineering and Primary Investigator of the Tissue Microfabrication Lab at Boston University. Chen is Associate Director of Research for the Center for Engineering MechanoBiology.
Prof. Chen’s research interests include the application of microfabrication and nanotechnology to cell and tissue engineering, and regenerative medicine. His lab has developed approaches to control the nanoscale adhesive interactions between cells and their surrounding scaffolds, and uses them to control cell function. Prof. Chen is particularly engaged in understanding how to engineer stem cell function and tissue vascularization, and the relationship between tissue architecture and tissue function.
Keywords: cell interaction with environment and subsequent effect on cell function; cell signalling; angiogenesis; stem cell biology; cell adhesion; cell mechanics; micro- and nanofabrication tools; molecular tools; mechanobiology
Paul Janmey
IRT 1 Co-Leader
Our lab studies the physical properties of fibrous networks and relates these properties to the mechanical responses of cells and tissues. In one project, we produce soft materials, usually hydrogels, to which cell adhesion proteins are linked to study how the stiffness of surfaces alters cell structure, function, and growth. Endothelial cells, fibroblasts, neurons and astrocytes each show unique dependence on both elastic and viscous properties of the substrate, and we seek to understand how they sense and respond to this mechanical cue. In related work we measure the structure and mechanics of cytoskeletal polymers using a variety of imaging, scattering, and rheologic methods. Further studies examine how changes in cell membrane structure mediated by inositol phospholipids lead to production of signals that remodel the cytoskeleton.
Faculty from a diverse array of institutions and scientific backgrounds, all contributing to research in mechanobiology.
Staff support research and education missions at the University of Pennsylvania and Washington University in St. Louis.
Postdoctoral fellows, graduate students, and undergraduates who have found success in many fields, including academia and industry.